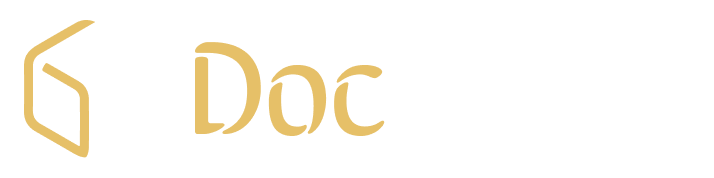Why add CAR-T to my portfolio?
For a growing Pharma company like yours, deviating from conventional line of new products is a challenge. If such a diversion is a “living product” all the more anxiety for your field force.
Add to that the complexity of convincing your Dr customers to start using such a therapy which requires huge expense on part of the patient with a rare possibility of loss of life due to its adverse events?
Answer from your marketing & sales team is anyone’s guess.

Context

However, if this Dr is a Hemato-oncologist understanding cutting edge therapies, and the patient is suffering from Leukemia where the survival rate in 5 yrs is less than 70%? If this patient is an adult, the survival rate comes down to 35% (in 5 yrs).
And if your new therapy is a single dose infusion of the patient’s own cells processed in a sophisticated lab to make its immune cells powerful enough to kill cancer cells? This changes the entire context as most Oncologists are aware about the revolution being created by this form of autologous therapy named “CAR-T” that is now in the global market for the last 6 years, marketed in India earlier this month with first patient treated.
MEDIA & SCIence JOURNALS
Positive reports
CAR-T is also being covered by the Indian media from time to time as seen from this article in TOI printed last year.
With the very 1st child in the world who received this therapy being in full remission after 10 years, this revolutionary treatment has crossed the proverbial Rubicon. Even an editorial from Journal of Clinical Oncology (shown in the adjoining box) called it a “cure” for B cell Acute Lymphoblastic Leukemia (ALL).

In India, ImmunoACT’s CAR-T product called NexCAR19 was approved by CDSCO few months back and the first patient was charged INR 42 lakhs. Their model is to directly approach hospitals and supply to their patients. Laurus labs has invested close to 100+ crores into this company in which they hold about 40% stake as per one of the TV interviews of Dr. Purwar (who heads ImmunoACT) given to a TV channel.
MECHANISM OF ACTION
What makes CAR-T a hero?
Understanding the basic problem in B cell cancers
When a person’s own immune system cant recognize cancer cells, the circulating immune cells in the form of T lymphocytes cant kill these allowing uncontrolled growth. In normal individuals, immune system recognizes an antigen on cancerous B cells called CD-19. Lack of such immune recognition of CD-19 antigen on the cancer cells leads to leukemias and Lymphomas.
What training and indoctrination converts the same T lymphocytes as commandos who identify and kill the cancer cells?
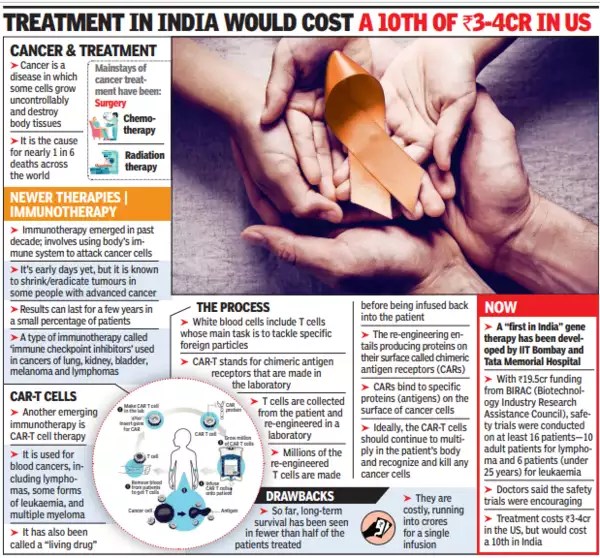
These inert T cells of the cancer patient can be genetically reprogrammed to recognize the CD-19 antigen and capture and attack it. The adjoining photo micrograph shows an actual activated CAR-T cell which has grabbed a cancer cell in the process of killing it.
Forced metamorphosis of inert T-cells into commandos

CAR-T therapy involves processing these inert T cells of the patient with a hybrid (hence Chimeric) fusion protein (T cell receptor fused with part of CD-19 antibody) that has been specially created in the lab and transferred to T cells of the patient outside, in a CAR-T cell factory.
This artificially constructed part (CAR construct) consists of an “antigen recognizing domain” and a “T cell activation domain” structured in a single protein that is formed inside inert T cells by a process called transduction. This indoctrinates them like trained commandos specifically against CD-19 antigen to fight cancerous B cells. These are then grown to adequate nos in a GMP CAR-T facility and then transfused back into the same patient in an ICU to treat an adverse event called “cytokine storm” that can occur in half the patients. During this storm, cancer cells are attacked in an “ambush” resulting in death of all cancer cells leading to a complete remission.
Thus, infusion of a huge population of highly specific and targeted T cells of the same patient (hence autologous) is the best way to treat B cell cancers as compared antibodies made by a Biotech company. Secondly, using antibodies doesnt overcome the problem of antigen escape that occurs in such patients who have relapsed to 1-2 lines of treatments.

Since these commando cells that are infused as part of this therapy, can persist for years in the patient’s body, they will still recognize cancer cells if a relapse occurs later. Until 2017, before Kymriah (Novartis CAR-T) was approved, there was no other satisfactory treatment for those B-cell cancers which failed 2 lines of chemotherapy.
SEEING IS BELIEVING
CAR-T cell creation in action
SUNRISE SECTOR
Cell & Gene therapy : Fastest growing sector of the Life Sciences Industry
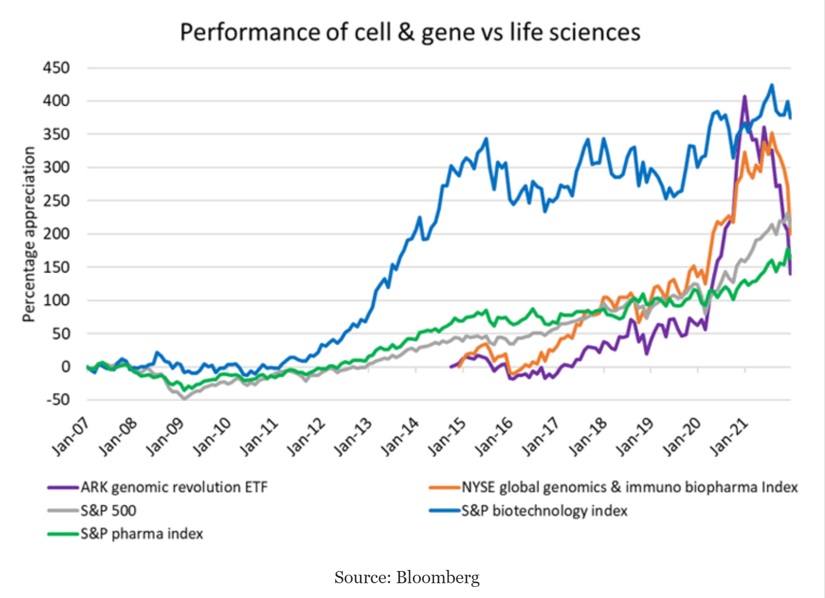
Conventional drug sales will grow at a compound annual growth rate (CAGR) of 6% from 2021 to 2026 and biologic sales excluding cell and gene therapies are forecast to grow from $415 billion to $541 billion, a CAGR of 5%.
Cell and gene therapies (CGT) in comparison, are expected to grow from $4 billion a year in sales to over $45 billion over that same period, a significantly higher CAGR of 63%.
More than 170 companies are working on CAR-T research programs with around 970 products in the pipeline. There have been over 260 collaborations between industry / academic stakeholders to develop these pipeline candidates. Over 6500 patents have been filed with respect to these products showing enormity of research being undertaken. In 2020, there were 474,519 new cases of leukemia and 627,439 new cases of lymphoma detected worldwide which are 2 main indications for CD-19 targeted CAR-T therapy. (Source: Root analysis, CAR T Cell Therapy Market- 4th Edition)
integration models
How complex is to start a CAR-T project?
This depends on whether you want to follow a forward integration model like ImmunoACT (a company spun off from IIT-B and ACTREC, funded by Laurus) as compared to a backward integration model of Immuneel Therapeutics (company floated by Biocon).
Forward integration model :
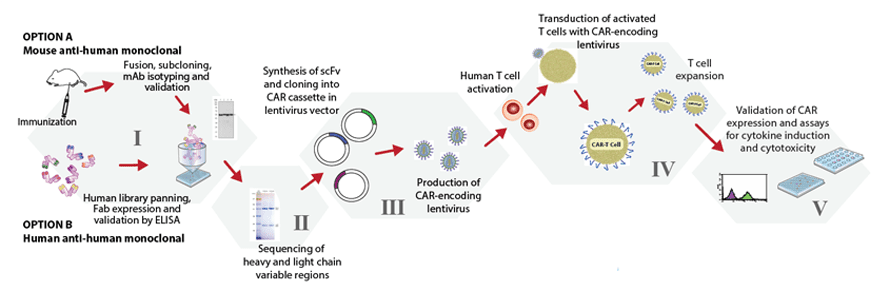
This involves creating your own CAR-T construct from scratch, patenting your sequences & process, generating regulatory data for each step for regulatory approval and reach clinical phase after many years on the bench.
This model would require creating a basic R&D facility with dozens of scientists working on genomics and the relevant expertise to make this long journey depicted above possible. Since the intellectual property scenario has hardly any precedents for court battles, may turnout to be a nightmare if challenged on IP front.
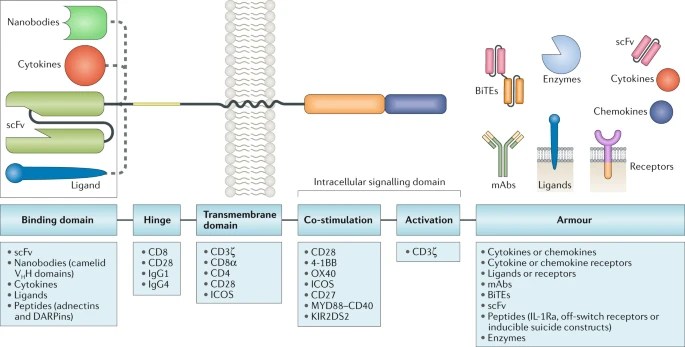
Variation of each of the above mentioned components of CAR construct development allows fine tuning of the functions and anti-tumour activity of the resultant CAR T cell product. CARs with various designs are being developed to improve the safety and efficacy of these therapies across various R&D labs and only high level expertise as well experience of commercializing these would permit creating these in India.
Backward integration model :
In this model, licensing of a CAR construct and its vector from an established company will expedite entry into the market. This can be licensed after thorough evaluation of in-vitro / in-vivo data and phase 1 clinical data to confirm that proof-of-concept is established. This data can be submitted to DCGI to request for a phase 2 pivotal trial in relevant indication that would seek a marketing authorization, after full data from such a pivotal trial in India becomes available.
With this model, market entry can be within a period of 2.5 to 3 years including pivotal clinical trial and approval with minimal risk of efficacy & safety. The pros & cons of both models can be discussed threadbare once interest is evinced.
In a backward integration model, how easy it is to set up a CAR-T facility?
In case you haven’t seen the video clip in an earlier section that shows the basics of how CAR-T cells are created, please click here to see it.
In the backward integration model, where CAR construct with the vector will be imported from an established CAR-T manufacturer, only facility for processing the T cell is required to be set up in India. This is akin to importing an API for formulating it in an Indian formulation factory.
For this purpose, there are either closed systems or open systems available since it involves multiple steps that can be done using different equipment. However, this field has been revolutionized by a German company called Miltenyi Biotec who created a “CAR-T facility in a box” shown in the video above. This equipment for making CAR-T was approved in 2018. This is a closed system which completes all the steps required for processing and growing T cells inside the machine in 14 days and packs the grown CAR-T cell population in a sealed bag at the end of the process, ready for shipping. Efforts are successful by Miltenyi Biotec to reduce this processing time to 7 days, though approval for this new process is pending. This entire GMP facility requires only 2500 Sq Ft area and a single trained operator to run it. There would be an additional QC lab of around the same area required for testing and validation of each batch before shipping.
CDMO Model for CAR-T:
Since many companies are not be interested in bearing total infrastructure cost for even this small set-up, we are working towards forming a consortium of companies who can split the cost of infrastructure/ development. A cell therapeutic facility in one of the metro cities has all the basic laboratory to house such a facility on contract basis. Details can be discussed in a face-to-face meeting for which following are contact details:
Dr. Dhananjay Bakhle (d.bakhle@outlook.com, +91-95455-36678)